MedTech Venture Builder Collaborative Centre of Excellence
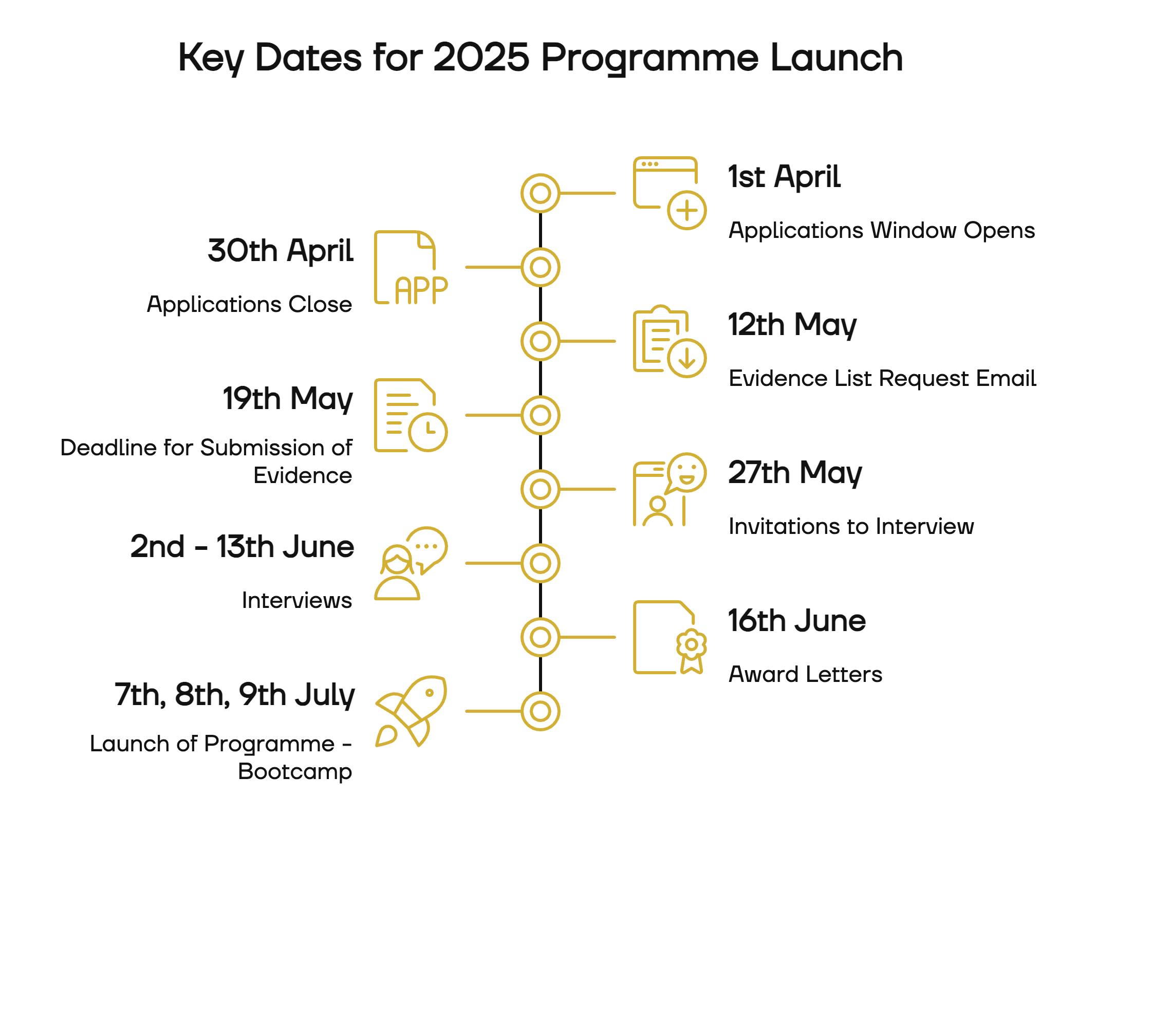
Starting July 2025 - Applications Closed
The MedTech Venture Builder (MVB), now in its 2nd year, is a 12-month structured programme in two stages to speed-up the commercial translation of promising medical technologies into robust, investment-ready healthcare ventures.
Supporting companies from across the UK, the MVB, the first of its kind, is a collaborative effort between London’s leading academic institutions, King’s College London (lead), Queen Mary University London, and City & St. George's University London.

Our collaborative focus on MedTech allows us to take ventures a significant step further than what has been achieved before programmatically.
We provide the structures that are critical to de-risking your company, such as a regulatory plan, a clinical evidence roadmap and stakeholder mapping, extensive hands-on guidance and monthly strategic progress review, all within a fit-for-purpose Quality Management System (QMS).
This is a truly unique opportunity for very select, high-potential MedTech companies from across the UK to receive fully customised, extensive executive support from experts in commercial translation from the partner institutions.
This is only possible with the support of a significant grant by Research England for a Collaborative Centre of Excellence (CCoE) for MedTech translation. The programme is further supported by our strategic partners, Team Consulting.


Space at the London Institute for Healthcare Engineering for up to 3 team members per venture, part-time, for the duration of the programme.
At the end of Foundation, only select participating companies will be invited to join the next stage.
For Execution stage costs, read the important information about this programme.

By participating in the MedTech Venture Builder your venture will:
The MedTech Venture Builder invites applications from:

LIHE is the latest trailblazing initiative from King’s College London


